
The Contamination Control Strategy (CCS) is a central concept in the 2022 EU GMP Annex 1 for Sterile Products, introduced to strengthen quality risk management for sterile pharmaceuticals. Earlier GMP standards relied heavily on terminal sterilization and sterility testing as primary quality assurance measures. However, advances in regulatory science and the Quality by Design (QbD) approach have highlighted the limitations of depending solely on final product testing. The revised Annex 1 makes CCS a mandatory requirement, emphasizing systematic contamination control from raw materials to the finished product. Companies are now expected to integrate multiple elements—personnel, equipment, processes, and environment—based on scientific assessment and risk analysis. This establishes a proactive, lifecycle-wide contamination prevention system, marking a clear shift in sterile drug regulation from a “post-production testing” approach to “end-to-end process prevention”.
01 Scope of Application
The CCS applies across the entire production lifecycle of all sterile drugs, including sterile preparations (such as injectables and ophthalmic products) and sterile active pharmaceutical ingredients (APIs). Its scope covers associated excipients, primary packaging materials, and production processes—such as formulation, filtration, and filling—as well as equipment cleaning and sterilization, environmental monitoring (including cleanroom classification and microbial/particulate control), personnel procedures, and gowning protocols. Additionally, CCS extends to supply chain management, including supplier audits, and facility design, such as cleanroom layout and airflow patterns, ensuring that every stage—from material receipt to product release—is systematically controlled. For certain non-sterile products requiring microbial load management in specific processes, CCS principles may also be applied as appropriate.
02 Control Objectives
The primary goal of the CCS is to minimize contamination risks—such as microorganisms, endotoxins/pyrogens, and particulates—through a multi-layered prevention approach. Key measures include:
1.Eliminating microbial contamination
To ensure sterile products are free of viable microorganisms, it is essential to control bioburden, maintain effective sterilization processes, and uphold strict aseptic conditions. This involves monitoring the bioburden of starting materials, thoroughly developing, validating, and periodically assessing sterilization processes, and continuously optimizing aseptic operations.
2.Controlling particulate contamination
To protect drug safety from both visible and invisible particles (e.g., glass shards, fibers), companies implement measures such as periodic process optimization, lifecycle management of critical equipment, appropriate garment selection, and quality assessments of raw materials and packaging components.
3.Preventing cross-contamination
Cross-contamination is mitigated through isolation technologies and validated cleaning procedures. Measures include unidirectional design of personnel and material flows and the use of isolators to ensure that different products or materials remain separate throughout production.
4.Ensuring endotoxin control
Endotoxin control is achieved through strict monitoring of water sources, raw materials, and equipment cleaning processes. The ultimate goal is to ensure that products consistently meet the quality standards of sterile, pyrogen-free, and particulate-free, supported by systematic defenses such as validated endotoxin removal procedures.
03 Development, Formulation, Evaluation, and Dynamic Management
1.Development and formulation
The development of a CCS should be grounded in scientific validation and risk analysis. Potential contamination sources—such as personnel operations, equipment interfaces, and environmental exposure—must first be identified. Control measures are then established through process validation, media fill simulations, and environmental monitoring data. For example, in the filling process, procedures should integrate air velocity assessments, particle monitoring, and personnel qualification training to ensure effective contamination control.
2.Evaluation
The evaluation phase involves regular audits to assess the effectiveness of the CCS. Trend analyses—such as microbial monitoring results and deviation investigations—are used to determine whether control measures are sufficient. Tools like FMEA (Failure Modes and Effects Analysis) can help identify weaknesses and guide optimization efforts.
3.Dynamic management
Dynamic management ensures that the CCS continuously evolves through a robust improvement cycle. When changes occur—such as process modifications, facility upgrades, or emerging risks—strategies are reassessed and updated to maintain a closed-loop system of monitoring, analysis, improvement, and validation. After identifying risks at each stage of the drug lifecycle, a risk-based management approach is applied. Control measures are prioritized according to risk level and fully integrated into all phases of production.
During implementation, the effectiveness and predictive value of these measures are regularly evaluated. Continuous assessment and review help identify areas for improvement, driving ongoing enhancement of the CCS. Refer to the figure below for an example of this process.
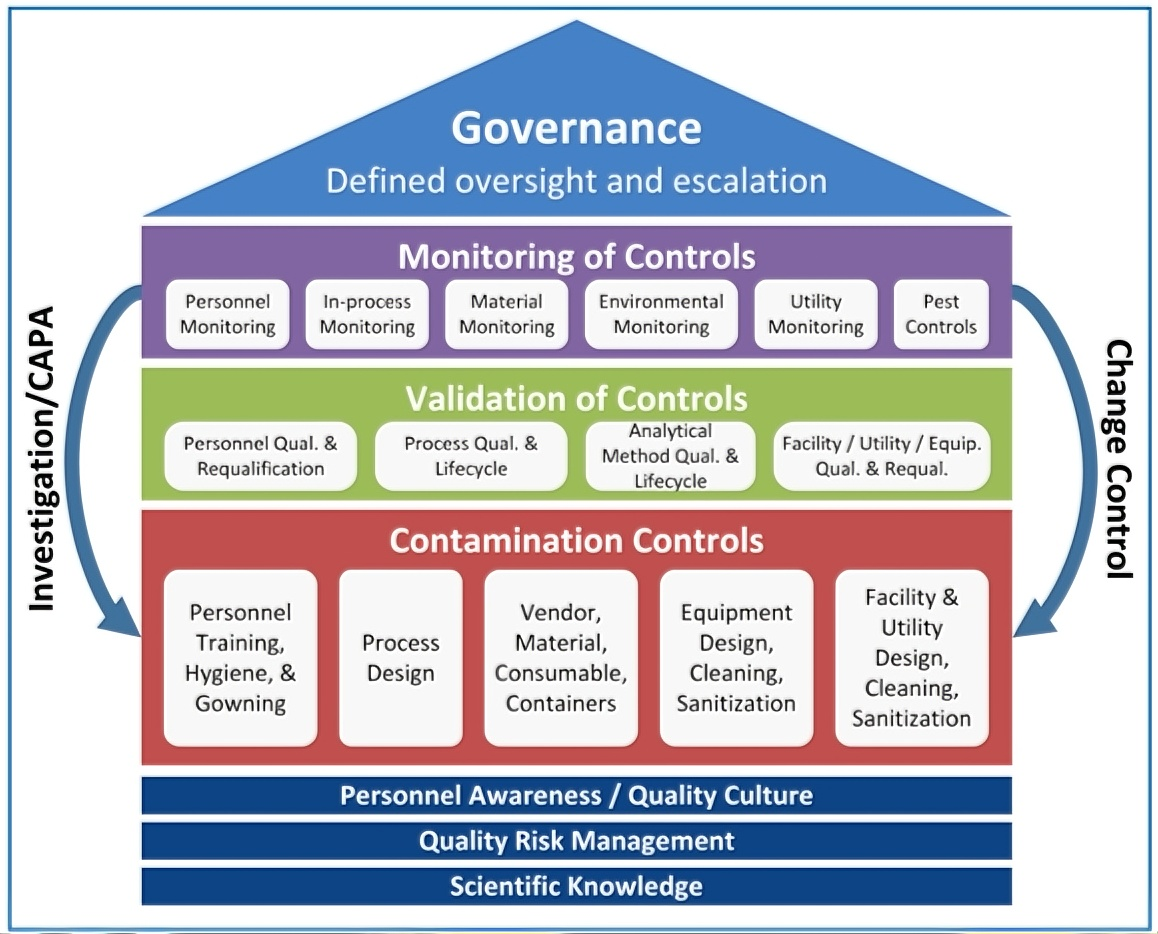
*Note: Each control element (shown in red) is designed based on foundational principles, validated to confirm it achieves the intended level of control (shown in green), and continuously monitored to ensure sustained effectiveness (shown in purple). The red-row elements do not represent an exhaustive list. Management (highlighted in light blue) reviews the outputs of each CCS element—such as monitoring data, validation results, investigations, and change controls—to ensure the overall integrity and effectiveness of the strategy. Any risk within an individual element, or misalignment between elements, can compromise the CCS and potentially lead to contamination.
Summary
EU GMP Annex 1 is driving innovation in sterile drug quality control by requiring the implementation of a Contamination Control Strategy (CCS). Companies are expected to take a holistic approach, establishing a proactive prevention network that integrates design, validation, and continuous monitoring to ensure consistent product quality and regulatory compliance. In March 2025, the Center for Drug Evaluation (CDE) of China’s National Medical Products Administration released a draft update to its GMP sterile drug annex for public comment, aligning with international regulatory standards while addressing domestic industry realities. Alongside China’s ongoing progress toward PIC/S membership, this reflects the rapid integration of the domestic pharmaceutical sector into the global regulatory framework. In this context, domestic pharmaceutical companies are encouraged to move beyond passive compliance and adopt a proactive approach, leading the way in implementing global standards and best practices to achieve stronger, more sustainable development.
References
[1] (Pharmaceutical and Healthcare Sciences Society, PHSS) Control Strategy White Paper-In Manufacture of Sterile Pharmaceutical/ Drug Products.-2014
[2] Parenteral Drug Association(PDA)PDA-TR90:Contamination Control Strategy Development in Pharmaceutical Manufacturing.-2023
[3] T/CPAPE 01-2024: Technical Guidelines for Contamination Control Strategy (CCS) in Aseptic Pharmaceutical Production
[4] NMPA-GMP 2025: Annex for Aseptic Pharmaceuticals (Draft for Comments)
About Morimatsu LifeSciences
Morimatsu LifeSciences is a key business segment of Morimatsu International Holdings Limited (Stock Code: 2155.HK). It comprises Shanghai Morimatsu Pharmaceutical Equipment Engineering Co., Ltd., Morimatsu (Suzhou) LifeSciences Co., Ltd., Shanghai Morimatsu Biotechnology Co., Ltd., Shanghai Mori-Biounion Technology Co., Ltd., Shanghai Morisora Technology Co., Ltd., Bioengineering AG, Pharmadule Morimatsu AB, and its affiliated companies.
Morimatsu LifeSciences is dedicated in providing core equipment, process systems, and smart modular facility solutions, and services for the pharmaceutical, biopharmaceutical, medical aesthetics, and fast-moving consumer goods (FMCG) sectors including (cosmetics, food, and health supplements), as well as data centers.
Our team comprises highly experienced professionals with deep expertise in process R&D, engineering design, advanced manufacturing, compliance and validation consulting, production execution, and intelligent operations. With broad experience across diverse industries, we fully understand the unique characteristics and process requirements of various products. This enables us to deliver tailored, end-to-end process solutions from the conceptual design stage, precisely aligned with client’s specific needs.
Morimatsu LifeSciences has established a strong global presence, supported by advanced R&D centers, design hubs, and state-of-the-art manufacturing facilities worldwide. Our well-established service network spans Europe,USA,Asia-Pacific, and emerging markets. We have successfully delivered outstanding, customized solutions to clients in over 40 countries and regions, gaining extensive experience in international project execution.
As a multinational enterprise with core strengths in process technology, modular facility construction, and intelligent manufacturing, Morimatsu LifeSciences is dedicated to meeting the evolving equipment and system needs of our key industries. Through continuous innovation and technological advancement, we are steadily expanding our global footprint, driving our international strategy forward, and delivering Morimatsu’s expertise, reliability, and innovation to the global life sciences and related sectors.