
In recent years, the National Healthcare Security Administration (NHSA) has mandated a focus on “Scope Expansion and Quality Improvement” for centralized procurement. With the introduction of the tenth batch of national centralized procurement, the total number of drugs subject to national and provincial centralized procurement has now surpassed 500. This volume-based procurement approach has intensified price competition, with average price reductions of around 50% for national centralized procurement drugs. Consequently, the procurement scale for these drugs has surged.
The continued push for centralized procurement is presenting pharmaceutical companies with both significant challenges and opportunities. Companies are now required to adjust their production models, focusing on fewer drug varieties and larger production capacities. This shift places higher demands on the stability and efficiency of their production processes.
Currently, most high-capacity granulation systems operate at around 200 kg per sub-lot. However, with the pressure from centralized procurement, there is an urgent need for granulation lines with capacities of 500 kg per sub-lot or more. Through extensive collaboration and discussions with various pharmaceutical companies, Morimatsu has identified several key areas for optimization.
Feeding Process
For feeding 100 kg of raw materials, vacuum feeding is an effective way to minimize manual labor. To boost feeding efficiency, it’s crucial to streamline the process, increase the material transfer rate, and reduce leaks in the vacuum system. We recommend using the granulation pot directly as the feed bin, as illustrated below. By employing a high-efficiency vacuum source and selecting multi-layered, folded filter materials, you can significantly reduce pressure loss. Additionally, reinforcing the pot’s seal will enhance the vacuum system’s ability to maintain negative pressure.
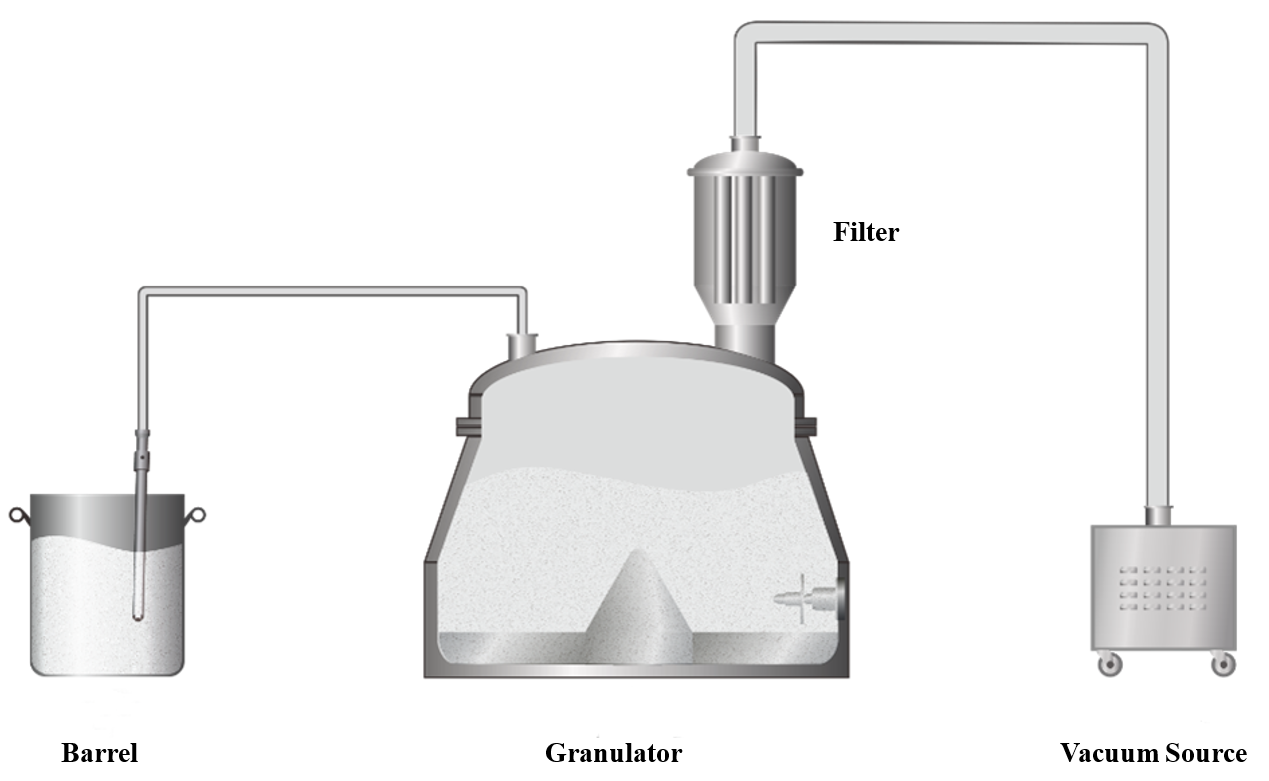
Granulation Process
In large-scale production, handling substantial volumes of raw materials can lead to challenges with mixing and granulation uniformity. As equipment size increases, issues such as dead zones and material residue become more pronounced. To address these challenges, we use high-power, high-shear impellers along with a tested and optimized pot design to achieve effective powder mixing with low RSD (Relative Standard Deviation) in a short amount of time. We also fine-tune the processing by maintaining the gap between the blades and the pot's bottom plate and side walls within 1 to 5 mm. Additionally, we ensure that the pot's inner wall is polished to a smoothness of Ra~0.2 μm. These measures help minimize mixing dead zones and reduce material residue during batch discharge.
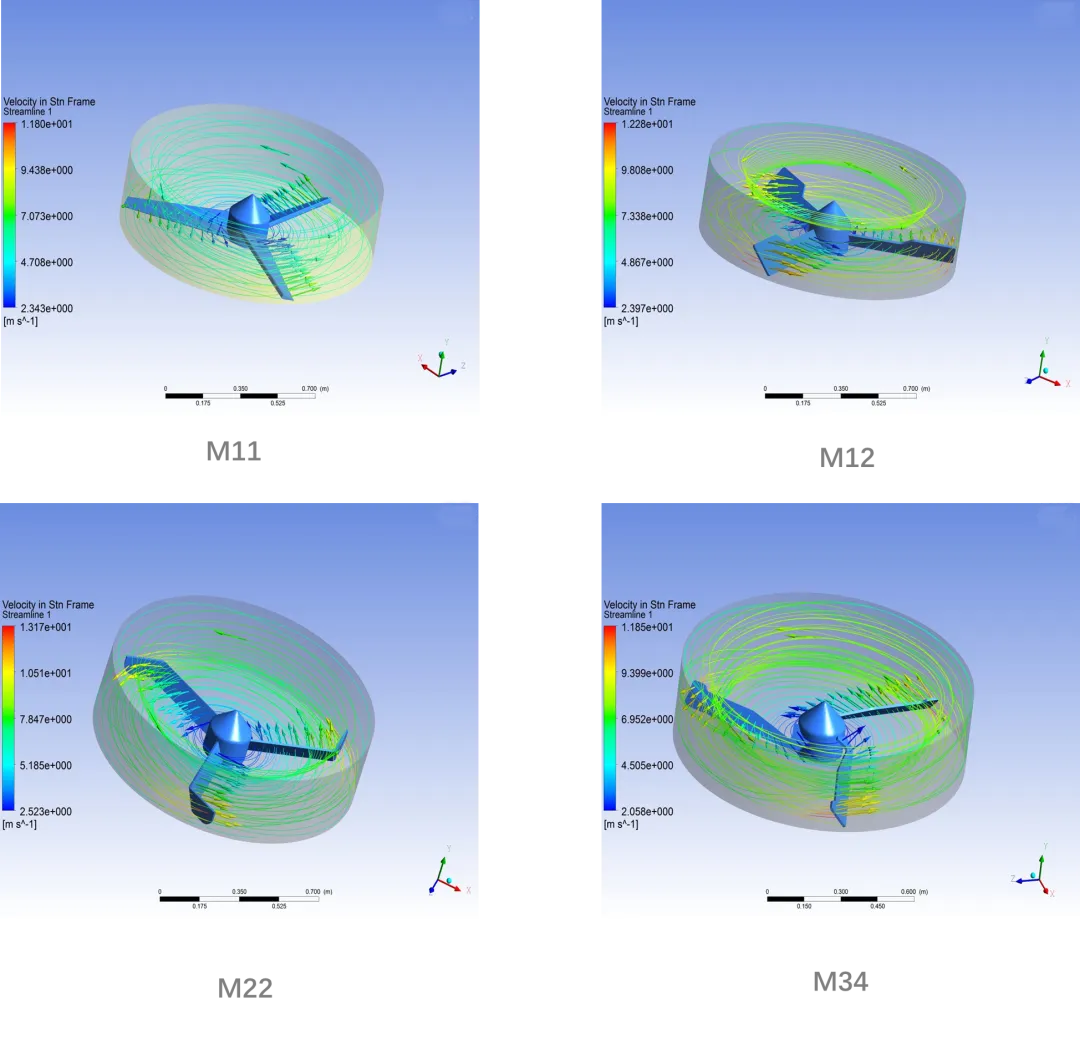
Flow Field Simulation of Shearing Effects with Various Blades
Wet Granule Transmission
In the context of centralized procurement and ongoing pharmaceutical innovation, we face larger batch sizes and increased frequency of sticky materials, which challenges the reliability and efficiency of the closed transmission system between granulators and fluidized beds. To address these challenges, we have increased the torque of the wet granulator, optimized the feeding pipeline, and added enhancements to the air supply and push devices. We've also refined the automatic control program for push speed and discharge valve operation to ensure both reliability and efficiency in the feeding process.
Moreover, large-scale production has minimal tolerance for faults, requiring a more user-friendly and efficient automatic control system with reduced manual intervention. Morimatsu precisely manages key parameters in both production and cleaning processes, provides hands-on training, and offers comprehensive batch production setup solutions, ensuring robust safety and efficiency in production.
Test Case
Morimatsu, in collaboration with a pharmaceutical company, conducted joint testing on the production process for various amine drugs using the optimized measures described above. For the viscous material in question, 300 kg of raw material was fed into the system in just 8 minutes, with the wet granule transfer completed in 10 minutes. Both the product’s RSD (Relative Standard Deviation) distribution and the granulation pot residues met the required standards, significantly enhancing the efficiency of the entire granulation line.
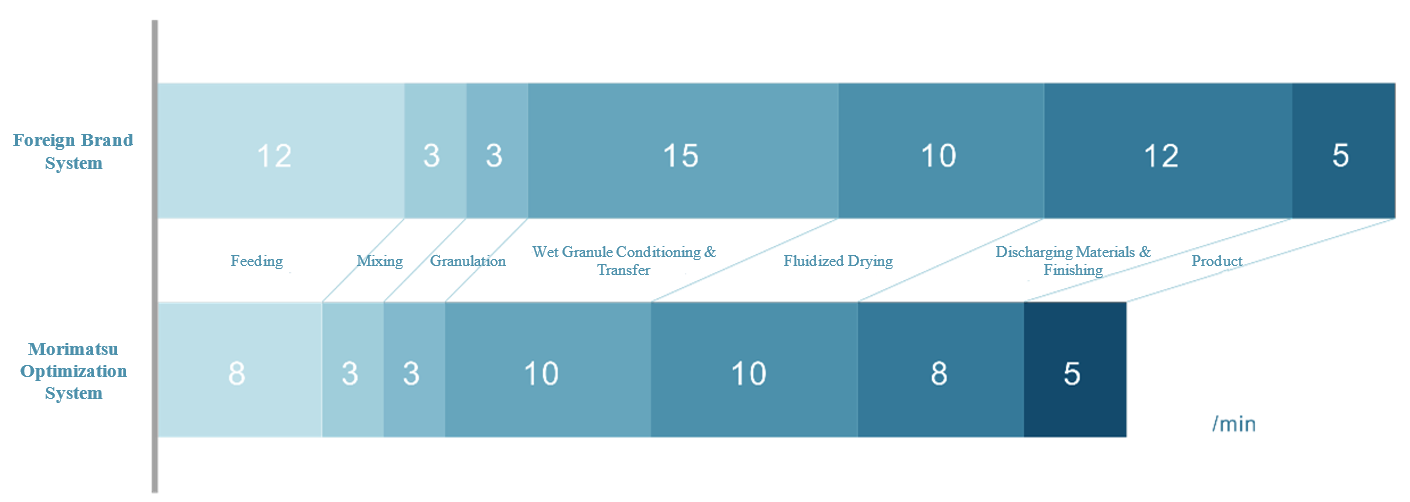
Comparison of Time Efficiency: Foreign Brand Granulation Line vs. Morimatsu Optimized Granulation Line
In the context of large-scale capacity expansion driven by centralized procurement, optimizing and improving processes and management across multiple stages is essential. Morimatsu, with its extensive technical expertise and industry innovation, has a sharp understanding of market trends. By accurately assessing customers’ production processes and needs, Morimatsu provides customized, high-quality solutions that consistently deliver greater value.
About Morimatsu LifeSciences
Morimatsu LifeSciences, one of the key business segments of Morimatsu International Holding Co., Ltd. (Morimatsu International, stock code: 2155.HK), mainly consists of Shanghai Morimatsu Pharmaceutical Equipment Engineering Co., Ltd., Morimatsu (Suzhou) LifeSciences Company Limited, Shanghai Morimatsu Biotechnology Co., Ltd., Pharmadule Morimatsu AB (Sweden) and its subsidiaries, which serves the pharmaceuticals, bio-pharmaceutical, cosmetic medicine, FMCG (cosmetics, baby, women & home Care, health care, fabric & home care, food, beverage, nutraceuticals) and other industries, providing customers with "core equipment+value-added services+digital intelligent overall factory solutions and services" ("MVP Solutions&Services"), focusing on core equipment, stainless steel process systems, disposable process systems, consumables, laboratory solutions, digital and modular factory solutions and services.
As a diversified multinational company, Morimatsu has opened subsidiaries or advanced manufacturing plants in China, Japan, Sweden, United States, India, Italy, Singapore, and has delivered different forms of products and services to more than 40 countries and regions so far, by its global footprint of an efficient and professional team.
Forward-Looking Statements
The information in this press release may include some forward-looking statements. Such statements are essentially susceptible to considerable risks and uncertainties. The use of "predicted", "believed", "forecast", "planned" and/or other similar words/phrases in all statements related to our company is to indicate that the statements are forward-looking ones. Our Company undertakes no obligation to constantly revise such predicted statements.
Forward-looking statements are based on our Company management's current perspectives, assumptions, expectations, estimations, predictions and understanding of future affairs at the time of the making of such statements. Such statements are not guarantees of future development and are susceptible to the impact of risks, uncertainties and other factors; some are beyond the control of our Company and unpredictable. Subject to the influence of future changes and development in our business, competition environment, political, economic, legal and social conditions, the actual outcomes may differ significantly from the information contained in the forward-looking statements.