
Introduction
With the development of targeted therapeutic drugs, more and more drug conjugates show excellent clinical results, and the curtain of the pan-coupling era has been quietly opened. Antibody-drug conjugates (ADC), peptide-drug conjugates (PDC), small molecule-drug conjugates (SMDC), antibody fragment-drug conjugates (FDC), immunostimulatory antibody drug conjugates (ISAC), radionuclide-drug conjugates (RDC), virus-like drug conjugates (VDC), antibody-siRNA conjugates (ARC), and antibody cell drug conjugates (ACC) have led people to believe that "all things can be conjugated" no longer stay in the imaginary stage. Due to the excellent performance of ADC, according to incomplete statistics, 41 out of every 100 drug conjugates under development belong to ADC, indicating the status of ADC in drug conjugates. This article will introduce the characteristics of ADC, and explore how to use engineering technology to achieve safe and efficient production of ADC, so that the "magic bullet" magic doubled.
Development history of ADC
As early as 1913, German scientist Paul Ehrlich proposed the concept of magic bullet, the basic idea of which is to selectively deliver toxins to the tumor site. However, limited by the technical level at that time, this concept still exists only in the hypothesis stage. It was not until 1958 that studies at the cellular level were started. In 1975, ADC began to be used in the study of animal models. In 1983, ADC began to be applied in clinical trials. In 2000, the FDA accelerated approval of the world's first ADC (trade name Mylotarg) for marketing. However, Mylotarg had to be withdrawn from the market in 2010 due to significant side effects. In 2011, the global second ADC (trade name Adcetris) was approved by the FDA for marketing, which is nearly a century away from the introduction of the ADC concept. By the end of 2018, there were only five ADCs marketed worldwide. The following years saw a surge in commercialization of ADCs from 2019 to 2022 as antibody technology matured. As of June 2022, 14 ADCs have been marketed: Adcetris®, Kadcyla®, Besa®, Mylotarg®, Polivy®, Padcev®, Enhertu®, Trodvy®, Blenrep® and Zynlonta™, Lumoxiti, Akalux, DisitamabVedotin (爱地希®), Tivdak. According to The Oncology Market for Antibody - Drug Conjugate (Carolina do Pazo et al), published in Nature Reviews, the market size of the first 10 ADC drugs is expected to exceed $16.4 billion by 2026.
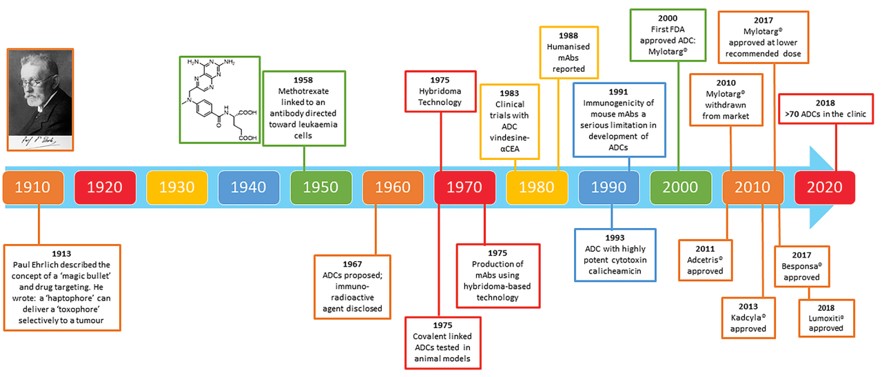
Image source: Cyjugic Payloads for Antibody – Drug Conjugates
In more than 100 years of development, ADC has undergone three generations of technology changes. Early ADC, due to immature technology, poor efficacy, strong toxic side effects and narrow therapeutic window, belongs to the first generation of ADC, and the representative product is Mylotarg. With the maturity of antibody technology and the improvement of conjugate technology and linker, the efficacy of the second-generation ADC has been improved, the toxic side effects have been reduced, and the treatment window has been wider. However, there is still a problem of random distribution of drug conjugates, and the representative products are Adcetris, Kadcyla, etc. In order to further improve the efficacy and reduce toxic side effects, the third generation of ADC using site-directed conjugate technology and new cytotoxics emerged, and the representative products are Enhertu and Trodvy.
Structure and mechanism of action of ADC
In the development history of ADC for more than 100 years, only 14 drugs have been successfully marketed, and their development difficulties are self-evident. Let's take a look at the structure and mechanism of the ADC.
ADC drugs usually contain the following three key components: antibodies, linkers, and small molecule cytotoxic drugs (Payload). These three components are also called the three elements of the ADC, as shown in the figure. The contents of ADC three-element and conjugate technology will be introduced in other articles.
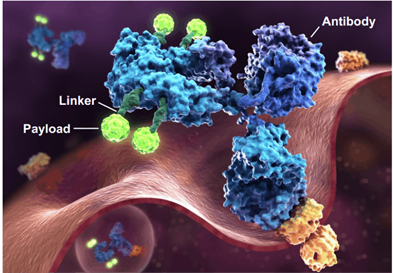
Three elements of the ADC, picture source: ADC REVIEW
How does an ADC that enters the blood work? It can be summarized into 6 steps:
01. The antibody component of ADC can recognize and bind to antigens on the surface of tumor cells
02. The ADC-antigen complex enters tumor cells by endocytosis
03. The ADC-antigen complex enters the lysosome, where the antibody is degraded
04. Cytotoxic drugs are released from lysosomes
05. Cytotoxic drugs destroy DNA or vascular bundles to inhibit the growth and division of tumor cells
06. Tumor cell death
ADCs with bystander effects can continue to attack other tumor cells after completing above effects.
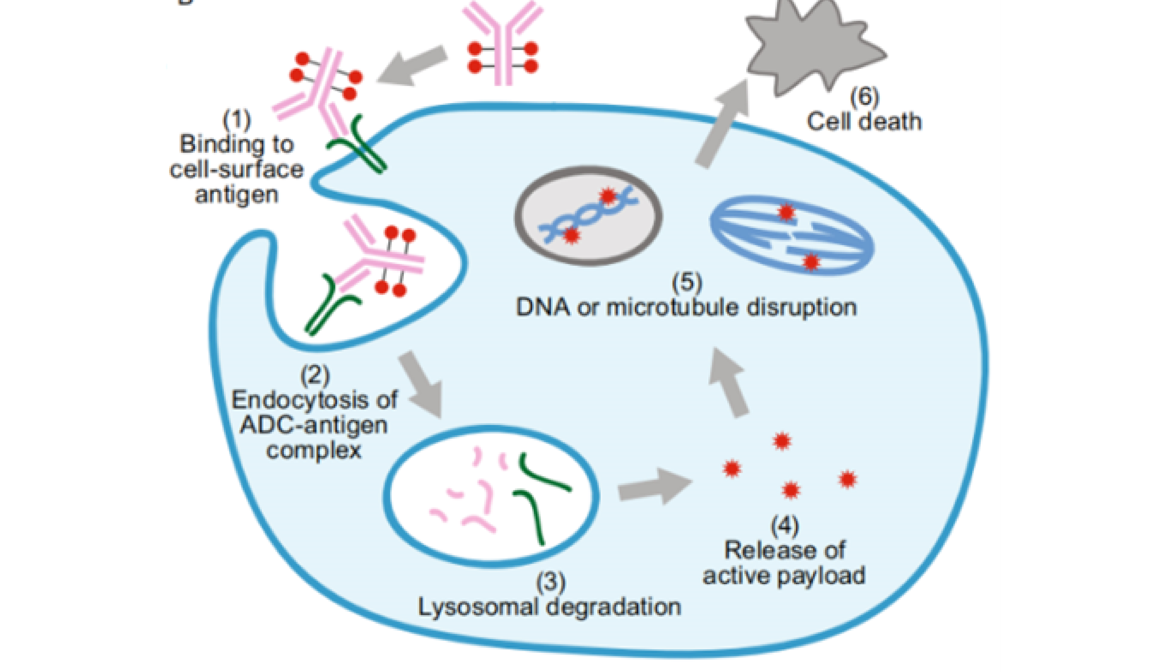
Image source: Protein Cell 2018, 9(1):33-46
ADC production difficulties and solutions
01 Complex production route and raw materials
ADC is a single molecule formed from a ternary complex. The production of ADC involves four parts: production of antibody, production of toxic drugs and linkers, production of conjugates, and preparation production, as shown in the following figure. These four parts have different requirements and are not minor challenges for production facilities, air conditioning systems and public engineering systems, material management, etc. For a pharmaceutical company, the difficulty and cost of production are much greater than those of ordinary macromolecules or small molecules. Therefore, some enterprises choose to produce ADC products in multiple habitats, or entrust some sections to CMO enterprises for production. The resulting compliance and supply chain management is also a major challenge for ADC drug production.
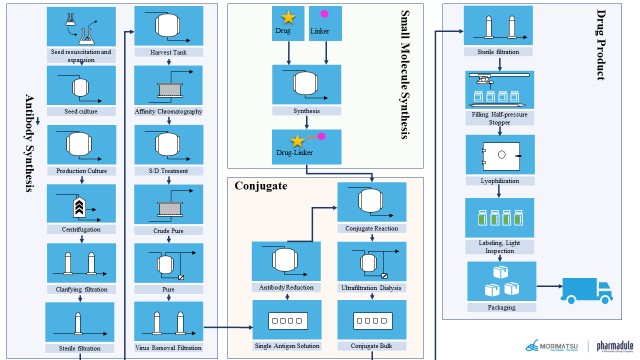
Image source: Typical ADC production flow
02 Safety risks caused by drug toxicity
For the cytotoxic drugs used by ADC, according to the requirements of National Institute of Occupational Safety and Health (NIOSH), the corresponding occupational exposure band (OEB) is OEB4 or OEB5, and the isolator needs to be used.
The Guidelines for On-site Inspection of Antibody Drugs (Draft for Comments) issued by China Food and Drug Administration divides toxic and harmful solid and liquid OEBs into six bands. Solid substances with OEB4 and higher should be handled in the isolator; operations involving liquids containing OEB4 and higher should be carried out in closed containers/systems. From the perspective of safety protection and sterility assurance, ADC preparations are preferably produced in the isolator. Appropriate protective devices should be selected for personnel in contact with ADC products according to OEB. Personnel should be gowned to minimize physical exposure (if isolator technology is used, attention should be paid to the airtightness of the isolator and the integrity test of gloves before and after use; the air return line of the isolator should be cleaned regularly, and it should be considered that there should be a cleaning device inside the isolator, and the isolator door should be opened after cleaning inside the isolator at the end of production). Personnel handling product exposure should undergo regular training and assessment.
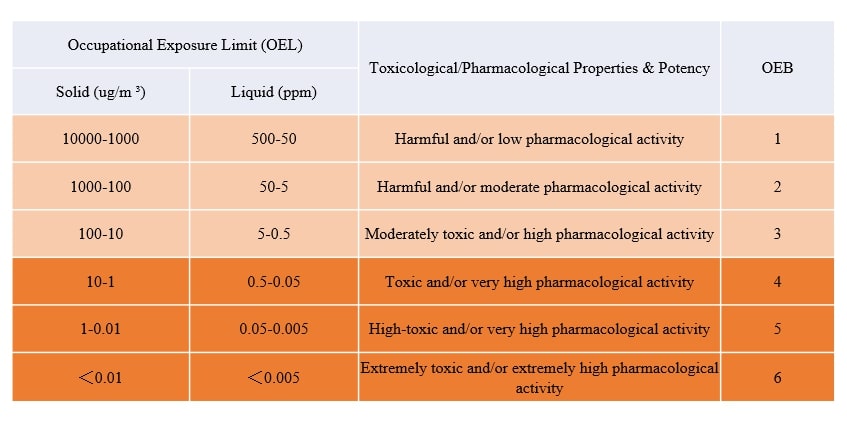
Table OEB and OEL Guidelines for On-site Inspection of Antibody Drugs (Draft for Comments)
03 Control of product quality attributes
As ADC has both antibody and small molecule cytotoxic drug production sections. Each section has its own unique critical quality attributes (CQAs). The quality attributes of antibody production section and small molecule cytotoxics should meet the quality attributes of antibody drug production and small molecule drug production, respectively. In addition, different from other macromolecular biological drugs, ADC drugs have unique heterogeneity, and ADC drugs with different Drug-to-Antibody Ratio (DAR) values reflect different clinical effects of drugs. The critical process parameters/steps and process parameter ranges should be determined based on the impact on the CQAs of ADC drugs, and the quality control strategies of intermediates, crude products and final products in the production process should be determined with a deeper understanding of products and processes and accumulation of production experience to ensure the consistency between production batches, so as to ensure the safety, effectiveness and quality control of ADC drugs. The critical quality attributes of ADC include but are not limited to: drug-to-antibody conjugate ratio (DAR), drug distribution ratio (conjugate site), free small molecule drugs, molecular size variants (multimers or fragments), residual solvents, etc.
04 Product yield
According to the different conjugate techniques used in ADC production, the conjugate techniques can be divided into random conjugate technique and site-directed conjugate technique. Regardless of the conjugate technique used, unconjugate, mis-conjugate sites, mis-conjugate amounts, dimeric or multimeric by-products can occur during manufacturing, which can lead to a decrease in yield of the target product.. In order to control the product yield, using appropriate conjugate technology, appropriate antibody modification, and optimizing the conjugate reaction conditions will help to improve the yield of the target product.
Based on the in-depth understanding of product process and accurate interpretation of industry regulations, Morimatsu can provide digital intelligent overall engineering solutions including core equipment (Machine), value-added services and modular engineering (Plant) for ADC R & D, pilot and commercial production. The service coverage of the whole life cycle of Morimatsu includes front-end services such as joint R & D, process scale-up, feasibility study, basic design, conceptual design, and digital top-level design, to product production, as well as digital operation and maintenance, verification, and other back-end services after delivery, which better help to quickly realize process transfer, process scale-up, and industrialized production.
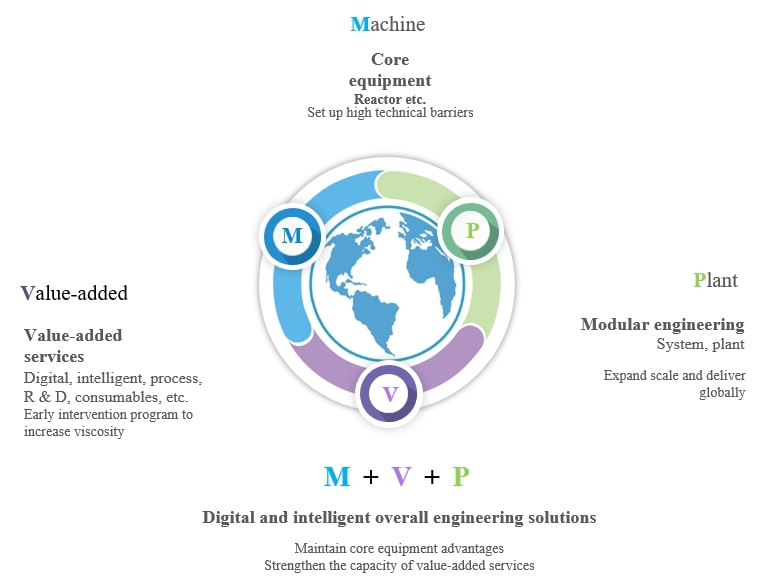
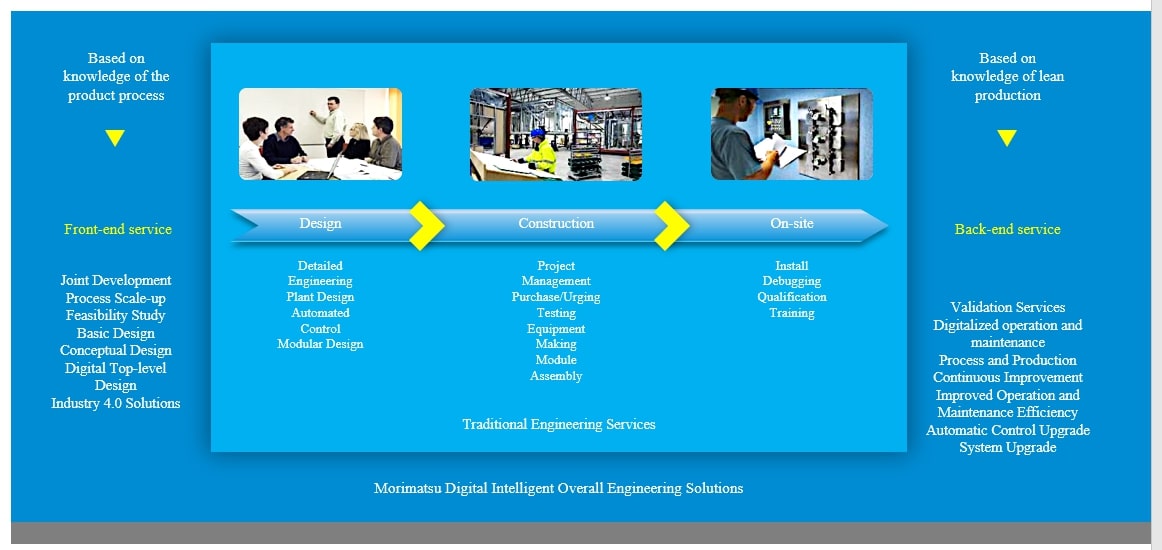
During the antibody preparation, small molecule production and conjugates production stages of ADC, the core equipment and modular system provided by Morimatsu are as follows:
Antibody Preparation
Bioreactor, medium distribution and storage system, buffer solution distribution and storage system, on-line liquid dispensing system, separation and harvesting system, purification system, concentration and filling system, biological wastewater inactivation system, etc.
Small Molecule Manufacturing
Reactors, temperature control system, etc.
ADC Conjugates Production
Desktop reactor, stainless steel reactor, disposable storage bag, liquid dispensing system, ultrafiltration system, isolator, etc., as well as the liquid dispensing and sterile filtration system required for preparations.

In addition, to help accelerate the marketing of products, Morimatsu’s modular factories can save the construction time of new factories and significantly reduce the risk of the construction process, while achieving the overall off-site relocation of the building, so that your R & D center and production base insert the "wings" where they need to fly.
Choose to work with Morimatsu to multiply the magic of your "magic bullet" ADC!
About Shanghai Morimatsu Pharmaceutical Equipment Engineering Co., Ltd.
Shanghai Morimatsu Pharmaceutical Equipment Engineering Co., Ltd (Stock Code: 2155.HK) is a wholly-owned subsidiary of Morimatsu International Holdings Company Limited. Morimatsu, founded in Japan and taken root in China, has developed into a multi-national company that embraces globalization, masters core technology, and gains rich experience from project implementation in diverse fields including core equipment, process systems, and engineering solutions. The company has built advanced manufacturing base in China, established affiliates in Sweden, the United States, India, and Italy, built an efficient professional globalization team, and delivered a variety of products and services to more than 40 countries and regions.
Forward-Looking Statements
The information in this press release may include some forward-looking statements. Such statements are essentially susceptible to considerable risks and uncertainties. The use of “predicted”, “believed”, “forecast”, “planned” and/or other similar words/phrases in all statements related to our company is to indicate that the statements are forward-looking ones. Our company undertakes no obligation to constantly revise such predicted statements.
Forward-looking statements are based on our company management’s current perspectives, assumptions, expectations, estimations, predictions and understanding of future affairs at the time of the making of such statements. Such statements are not guaranties of future development and are susceptible to the impact of risks, uncertainties and other factors; some are beyond the control of our company and unpredictable. Subject to the influence of future changes and development in our business, competition environment, political, economic, legal and social conditions, the actual outcomes may differ significantly from the information contained in the forward-looking statements.