
Introduction
COVID-19 mRNA vaccine was put into emergency use in just one year after the outbreak of the epidemic, which set a new record for the speed of vaccine research and development, provided enough protection to the people, and undoubtedly became the MVP in the epidemic control of COVID-19. MRNA drugs have attracted more attention from practitioners and investors, and have more opportunities to show its special charm.
Although COVID-19 mRNA vaccine was used urgently in just one year, its technology has a history of 60 years. In the past 60 years, scientists have overcome these difficulties before the theoretical possibility into the vaccine around us.
MRNA is a classic application of the central principle of biology, and the application of technology is mainly restricted by two aspects: sequence design, modification and delivery mode. In sequence design and modification, the sequence design is to get the target protein better, while the modification is to adjust the immunogenicity of mRNA, adjust the intensity of immune response, and protect personal safety. Delivery mode is the first problem that scientists realized. Because mRNA is a negatively charged macromolecule, it is unstable in the natural environment and cannot pass through negatively charged cell membranes. Therefore, there is a need for a ingenious technique that enables mRNA to be delivered into cells and restores protein translation function of mRNA in cells. Therefore, the delivery technology becomes critical, requiring a good balance of packaging efficiency, delivery efficiency, endosomal escape, toxic and side effects, stability, and other aspects.
Based on this, we will discuss the development of delivery technology, as well as the technical mechanism, quality analysis and preparation method of LNP.
Development of delivery technology
Delivery technology is a specialized subject, which has a history of 70 years from 1950 to now, and has gone through three stages: Drug Release Mechanisms, Nanomedicine, and Precision Medicine & Health Equity. Our current mRNA vaccines are in the transition stage from Nanomedicine to Precision Medicine & Health Equity.
The delivery technology of mRNA first started from protamine (1961), went through liposome encapsulation research (1978), cationic lipid application (1989), and FDA approval of LNP encapsulated SiRNA drug (2018). By the end of 2020, the formed LNP delivery technology was applied to Pfizer and Moderna's COVID-19 mRNA vaccine. The success of COVID-19 vaccine has made the public and the investment community pay more attention to the application of delivery technology.
The current research and application of mRNA and DNA-related delivery technologies mainly include the following: LNP (lipid nanoparticle), LPP (lipid polymeric complex), PNP (polymer nanoparticles), INP (inorganic nanoparticles), virus vector, exosome, and GalNac(N- acetylgalactosamine).
Similar principles are applied to LNP, LPP, PNP, and INP. The researches on LPP, PNP and INP are started with encapsulated materials, so as to obtain better adaptability and lower material manufacturing cost, and also to avoid the patent influence of LNP.
Virus vector technology is currently mainly applied to gene therapy due to the limited virus resources.
Exosome technology has attracted wide attention due to its more possibilities to simulate the physiological process of cells, but there are still many problems to be solved due to its engineering method.
The problem of cell membrane penetration is solved using GalNac technology. However, due to its penetration through chemical bond binding, it does not protect the mRNA molecule itself, so it is widely used for the delivery of siRNA and miRNA, but has not been effectively applied to the delivery of such macromolecules as mRNA for the time being.
In summary, the drug delivery technology of nanoparticles represented by LNP has become the mainstream technology for mRNA vaccine. Since the raw materials of LNP are either ingredients existing in the body such as cholesterol and phospholipid, or ingredients with large drug application background such as PEG-modified phospholipid and ionizable lipid, the acceptance of LNP is much higher than that of other several delivery technologies of nanoparticles when approved by FDA. In addition, the patent medicine precedent of COVID-19 vaccine and the large amount of safety data feedback brought by mass inoculation, the delivery technology of LNP is currently easier to be approved than other technologies.
However, the LNP delivery technology currently does not achieve targeting, so vaccines need not be targeted, as long as they can be effectively delivered to cells and express enough antigens. Once the mRNA drug treatment stage is reached, the corresponding schemes, either targeted modification or more drug aggregation to the lesion must be considered for LNP or other delivery technologies. The liver enrichment due to the nanoscale of LNP has attracted the attention of many scientists who study liver diseases. With the further study of these mechanisms, more detailed and specific requirements for the follow-up preparation technology will inevitably be put forward.
Next, we will learn more about LNP technology from the following aspects:
1. Mechanism of LNP technology
LNP (Lipid NanoParticle) is composed of four ingredients, forming a whole nanoparticle, which plays the function of protecting mRNA and penetrating cell membrane.
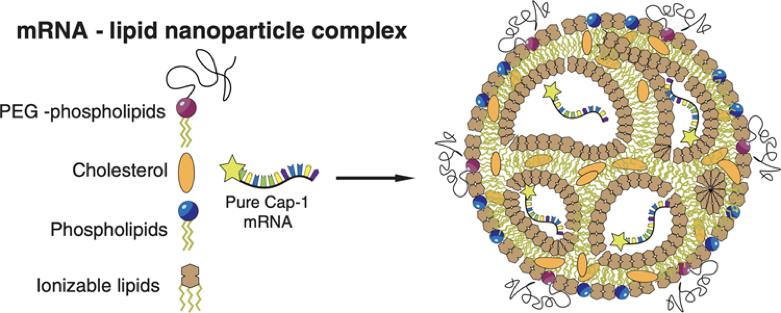
The following four ingredients have their own independent functions and complementary functions. When it comes to LNP, we usually talk about the overall structure formed by such a formulation.
1)Ionizable cationic lipid
PH-sensitive, capable of binding to negatively charged mRNA for efficient encapsulation and protonation in an intracellular acidic environment to facilitate intracellular escape, is a core part of the LNP design.
2)Distearoyl phosphatidyl choline (DSPC)
It is stable in normal environment, and in acidic environment, it can cooperate with cationic lipid to realize intracellular escape.
3)Cholesterol
Stabilize the structure of LNP particles, give the bimolecular film a certain fluidity, and resist external interference more.
4)PEG modified resin
Improve the stability of LNP, avoid premature elimination by human system, and give full play to the role of vaccine.
As shown in the ingredient proportions of the marketed vaccines below, the liposome ingredients are basically the same, with the main difference being the ionizable cationic lipid. The proportions of the ingredients in each formulation are also close to each other, so a stable structure is easy to be formed.
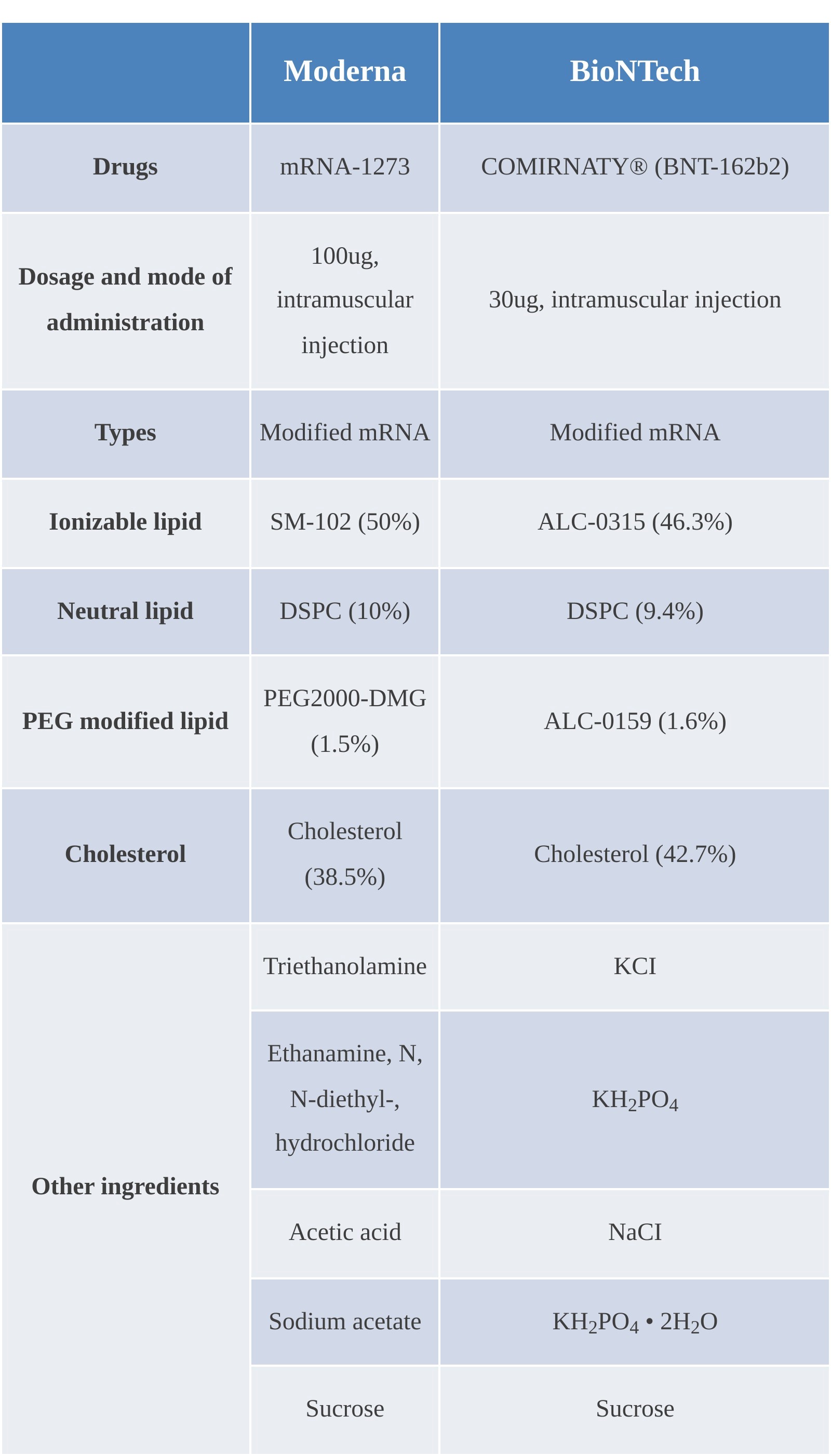
(Comparison of ingredients of marketed vaccines of Moderna and BioNTech)
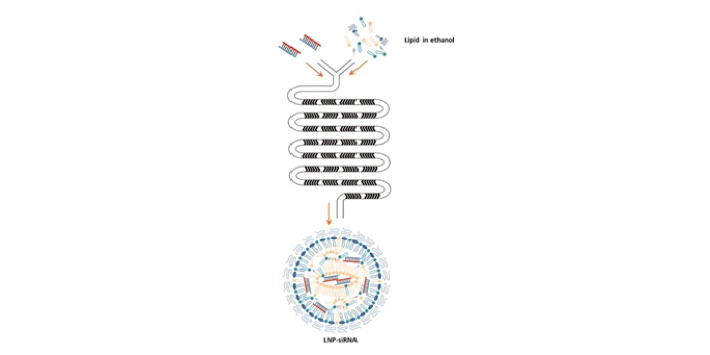
The reason why LNP is superior to traditional liposomes lies in its formation mechanism, which has the charge effect of ionizable lipids and mRNA. However, traditional liposomes need strong shearing or solvent evaporation to realize the coating and forming of drugs. Therefore, the formation of LNP is a mild and spontaneous process. The mild process of LNP also provides sufficient protection for mRNA.
- The mixed lipid-cholesterol/neutral auxiliary phospholipid/PEGylated phospholipid/ionizable lipid is dissolved in ethanol phase.
- The mRNA is dissolved in a buffer of aqueous medium (pH=4) (see the 2 buffer systems above).
- The aqueous phase and the ethanol phase are mixed, and during the mixing process, on the one hand, the ionizable lipids contact the acidic environment and exhibit proton property, and bind to mRNA under the action of static electricity; on the other hand, since the lipids enter the aqueous environment, they begin to self-agglomerate. Nanoparticles are formed in this way.
- PEG resin is involved in the generation of nanoparticles due to its spatial structure and the properties of PEG modified end, which helps to control the particle size.
Based on the principle analysis, how to quickly achieve the uniformity of the aqueous phase and the alcohol phase on a smaller scale is the focus of ensuring the preparation effect of LNP.
2. Quality analysis of LNP
The nanoparticles formed by LNP need to fulfill the required physiological functions in vivo and meet the following attribute requirements:
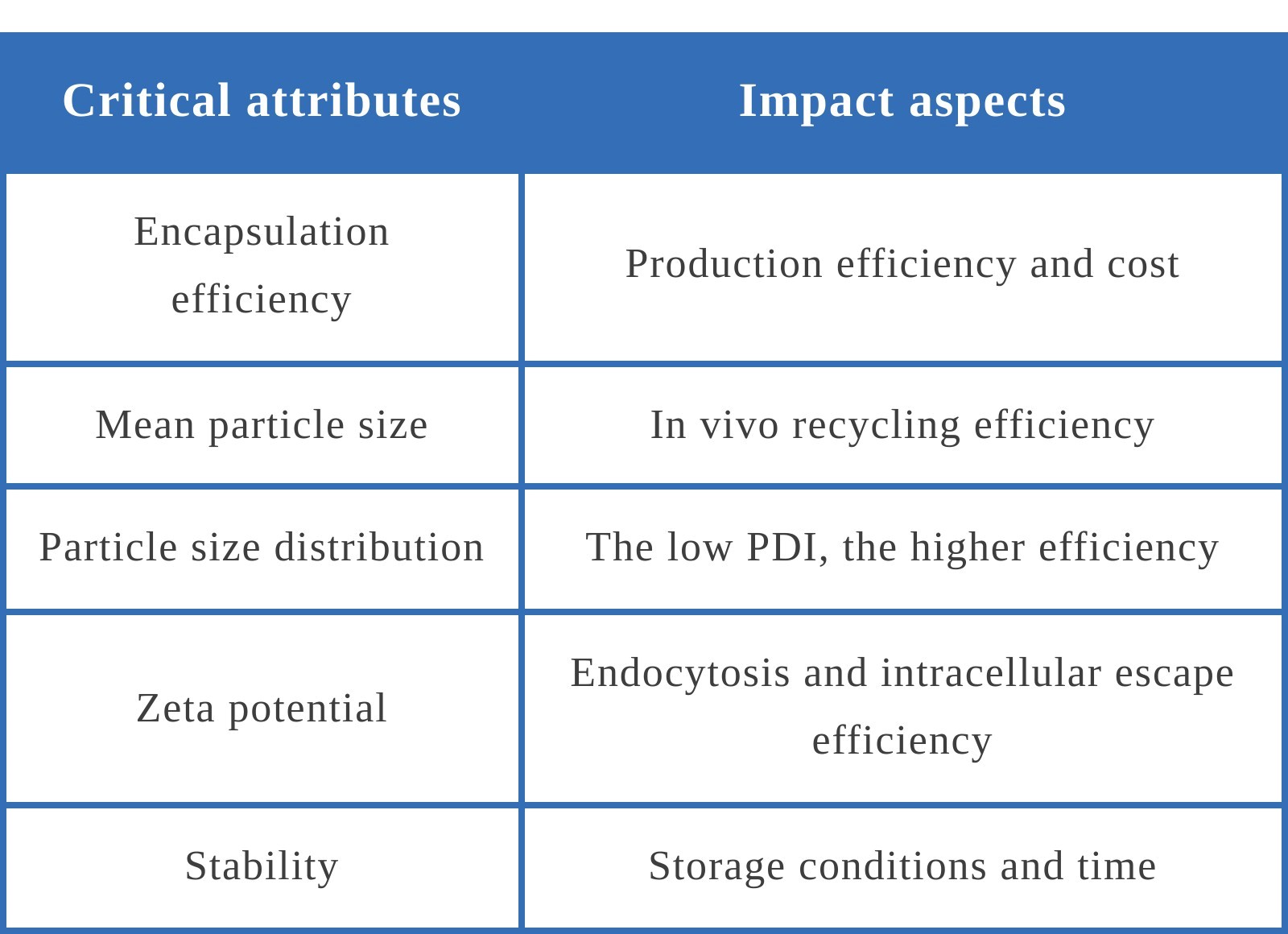
These attributes are more or less affected by the preparation process of LNP.
Encapsulation efficiency has been discussed much to date, but not the amount of mRNA contained in each particle. On the one hand, it is difficult to measure the quantity of mRNA; on the other hand, the effectiveness of vaccine depends more on the total quantity of mRNA, so the quantity of mRNA contained in a single particle is not the critical parameter like the DAR value of ADC drugs. Encapsulation efficiency, which can reflect the active ingredient content and preparation efficiency in the vaccine, has been used as a benchmark for quality measurement.
The discussion on storage conditions is an interesting topic. At present, some manufacturers require a storage condition of -70 to -80℃, some manufacturers require -20℃, some manufacturers require 2 to 8℃, and require different storage time. It can be seen that the slightly different formulation results in the difference of product stability. Since the LNP particles of mRNA are weakly bound spheres formed by the forces of hydrophobicity and charge attraction, they are easily broken. In addition, the particle size of LNP is about 100nm. On this scale, the influence of molecular thermal movement can no longer be ignored. The more stable the LNP nanoparticles are, the less stringent the storage temperature of the vaccine can be. The study of stability usually requires more research on formulation and preparation technology, and it also takes longer time to complete.
3. Preparation method of LNP
Starting from the preparation mechanism of LNP, various laboratories and scientific institutions have developed various mixing methods to realize the preparation of LNP. Several representative designs are shown below:
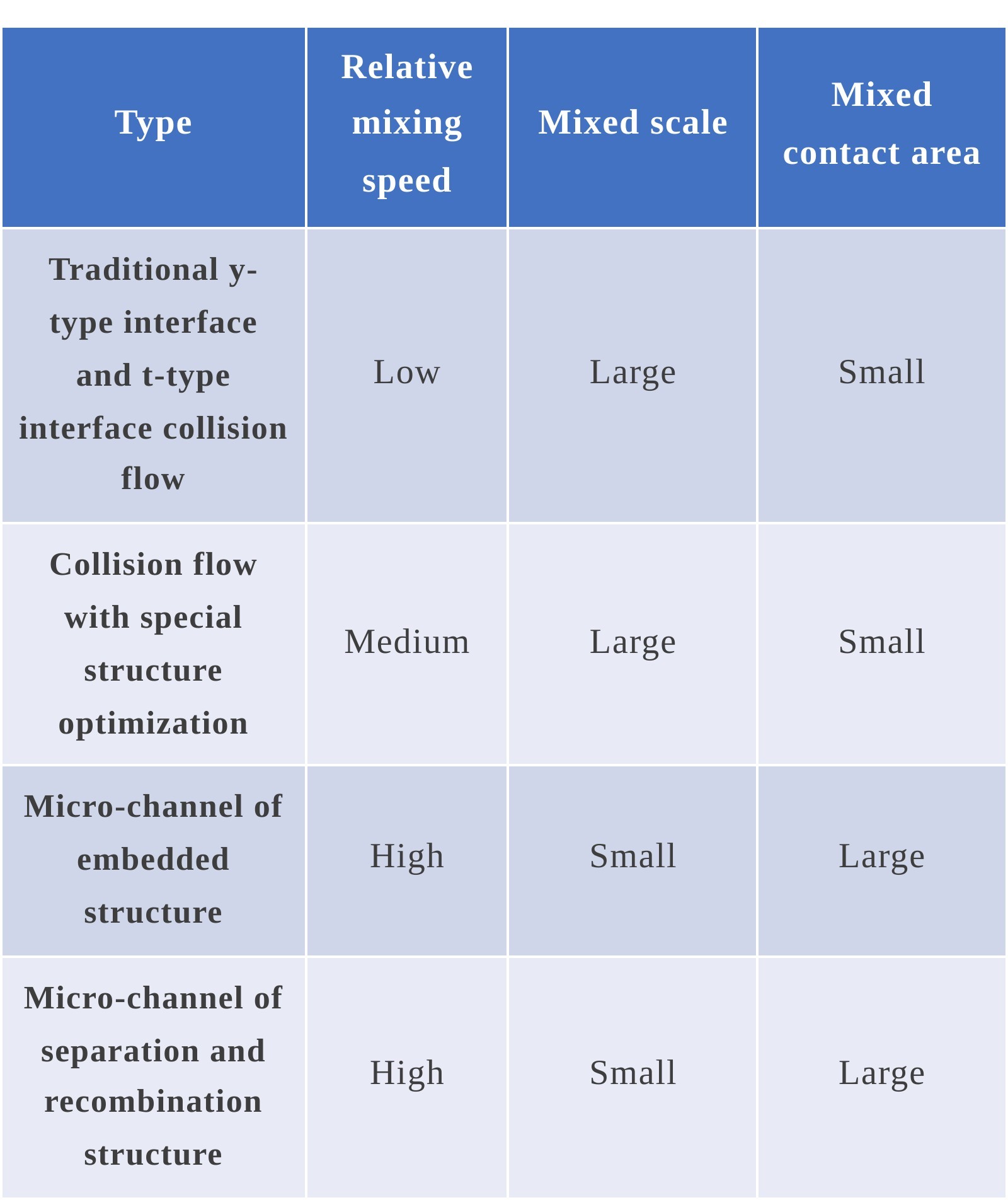
1) Design of traditional y-type interface and t-type interface collision flow
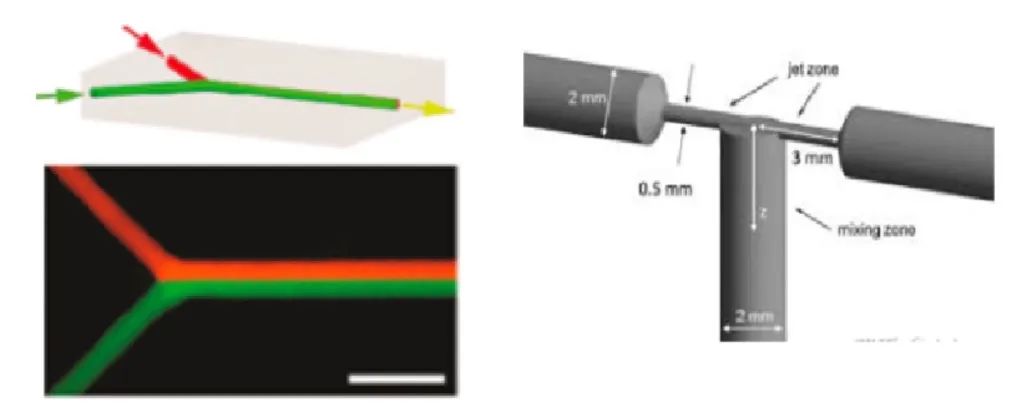
2)Design of collision flow with special structure optimization
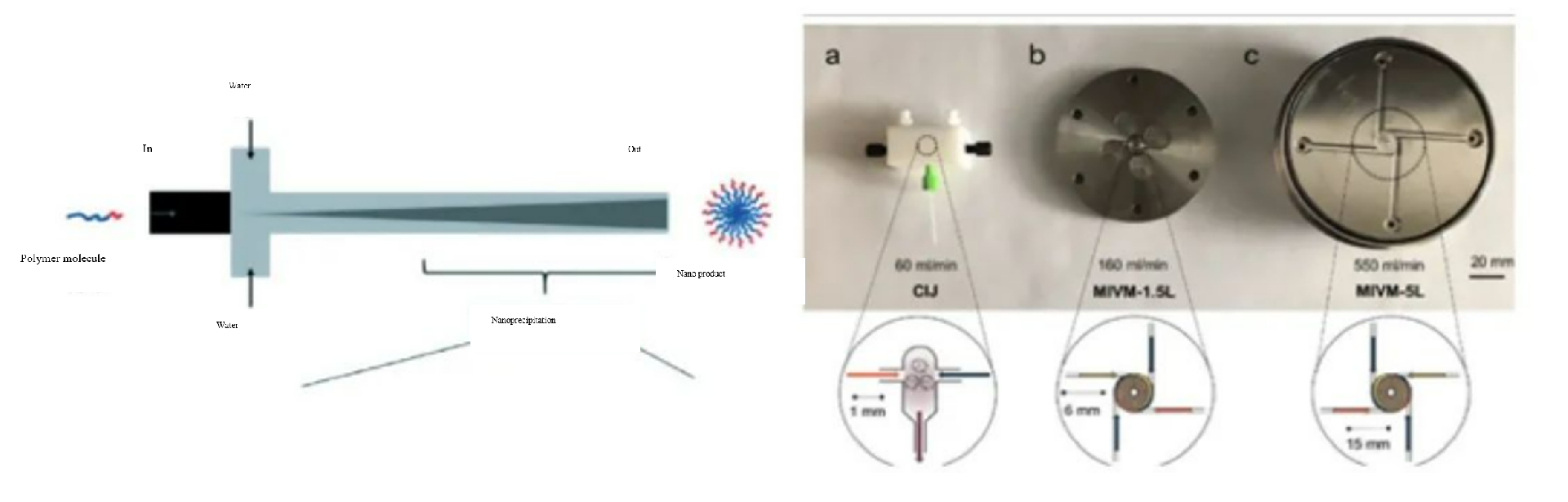
3)Micro-channel mixing technology by increasing disturbance of embedded structure
4)Micro-channel mixing technology of separation and recombination structure represented by Tesla structure
See the table below for the evaluation of mixing capacity versus several structures:
These mixed structures have been applied in the industry, and they can meet appropriate encapsulation efficiency and particle size control indexes under certain conditions. However, more tests are required to determine the mixed structure and final control parameters, which greatly affect the drug development time and development cost. In addition, there is no completely reliable physical model during amplification, resulting in serious waste during amplification.
However, with the further disclosure of LNP structure and further analysis of the formation process of nanoparticles, it is hoped that there will be a more clear direction to reduce the number and time of experimental exploration and improve the efficiency of R&D.
Prospects for the future
The development of mRNA drugs is more and more frequent, and we are constantly trying new delivery formulations and new raw materials. How the delivery system, especially the current mainstream LNP technology, respond to changes in formulations and raw materials is improving, and it has become a bottleneck in drug development and production, which is currently a critical aspect. Only the combination of basic theory, advanced tools and experiments can help the further development of LNP delivery technology.
Morimatsu continues to pay attention to the latest technological trends, and constantly pursues innovation and progress in the spirit of innovation and change. What we master is not only the ability of equipment and engineering construction, but also the in-depth exploration of product attributes and production process. And, we know that only when we really understand the critical quality attributes of the product, combine the production process and identify the critical process parameters, can we design and produce the production equipment to meet the needs of the product process.
Statement
The purpose of this article is to exchange industry technology and share knowledge with you, so as to promote common progress. Part of the content comes from the Internet. If there is anything wrong, please contact us and we will delete it in time.
About Shanghai Morimatsu Pharmaceutical Equipment Engineering Co., Ltd.
Shanghai Morimatsu Pharmaceutical Equipment Engineering Co., Ltd (Stock Code: 2155.HK) is a wholly-owned subsidiary of Morimatsu International Holdings Company Limited. Morimatsu, founded in Japan and taken root in China, has developed into a multi-national company that embraces globalization, masters core technology, and gains rich experience from project implementation in diverse fields including core equipment, process systems, and engineering solutions. The company has built advanced manufacturing base in China, established affiliates in Sweden, the United States, India, and Italy, built an efficient professional globalization team, and delivered a variety of products and services to more than 40 countries and regions.
Forward-Looking Statements
The information in this press release may include some forward-looking statements. Such statements are essentially susceptible to considerable risks and uncertainties. The use of “predicted”, “believed”, “forecast”, “planned” and/or other similar words/phrases in all statements related to our company is to indicate that the statements are forward-looking ones. Our company undertakes no obligation to constantly revise such predicted statements.
Forward-looking statements are based on our company management’s current perspectives, assumptions, expectations, estimations, predictions and understanding of future affairs at the time of the making of such statements. Such statements are not guaranties of future development and are susceptible to the impact of risks, uncertainties and other factors; some are beyond the control of our company and unpredictable. Subject to the influence of future changes and development in our business, competition environment, political, economic, legal and social conditions, the actual outcomes may differ significantly from the information contained in the forward-looking statements.